
SL Paper 3
Ethylamine, \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}}\), is a weak base.
State the equation for the reaction of ethylamine with water.
Explain why ethylamine has basic properties.
State the formula and deduce the shape of the positive ion (cation) formed when triethylamine, \({{\text{(}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{)}}_{\text{3}}}{\text{N}}\), reacts with hydrochloric acid.
Markscheme
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{N}}{{\text{H}}_{\text{2}}} + {{\text{H}}_{\text{2}}}{\text{O}} \rightleftharpoons {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{NH}}_{\text{3}}^ + + {\text{O}}{{\text{H}}^ - }\);
Accept \( \to \) in place of \( \rightleftharpoons \).
non-bonding/lone pair of electrons on the N atom (enables proton/\({{\text{H}}^ + }\) acceptance) / OWTTE;
\({{\text{(}}{{\text{C}}_2}{{\text{H}}_5}{\text{)}}_3}{\text{N}}{{\text{H}}^ + }/{{\text{[(}}{{\text{C}}_2}{{\text{H}}_5}{{\text{)}}_3}{\text{NH]}}^ + }\);
tetrahedral;
Examiners report
Majority of the candidates scored the mark for part (a). Lack of reference to N and lone pairs in part (b) penalized many candidates in scoring the mark. Most candidates were unable to explain the stronger basic properties diethylamine in part (c). Responses indicated weak understanding of the inductive effect of the alkyl groups. Many candidates did not address it the increased inductive effect due to two alkyl groups.
Majority of the candidates scored the mark for part (a). Lack of reference to N and lone pairs in part (b) penalized many candidates in scoring the mark. Most candidates were unable to explain the stronger basic properties diethylamine in part (c). Responses indicated weak understanding of the inductive effect of the alkyl groups. Many candidates did not address it the increased inductive effect due to two alkyl groups.
Majority of the candidates scored the mark for part (a). Lack of reference to N and lone pairs in part (b) penalized many candidates in scoring the mark. Most candidates were unable to explain the stronger basic properties diethylamine in part (c). Responses indicated weak understanding of the inductive effect of the alkyl groups. Many candidates did not address it the increased inductive effect due to two alkyl groups.
Steel is an alloy of iron, carbon and other metallic and non-metallic elements. Stainless steel contains about 18% chromium and 8% nickel. Explain why iron can form alloys with other transition metals.
Markscheme
their atoms/ions have similar/slightly different radii/size/densities/properties;
so do not disrupt crystal structure/metallic lattice significantly / OWTTE;
Examiners report
Part (b) was very poorly answered with most candidates providing general explanations of the formation of alloys.
Lanthanum, La, and antimony, Sb, form compounds with bromine that have similar formulas, LaBr3 and SbBr3.
Determine the type of bond present in SbBr3, showing your method. Use sections 8 and 29 of the data booklet.
Lanthanum has a similar electronegativity to group 2 metals. Explain, in terms of bonding and structure, why crystalline lanthanum bromide is brittle.
Markscheme
polar covalent
average electronegativity «= \(\frac{1}{2}\)(3.0 + 2.0)» = 2.5 AND electronegativity difference «= 3.0 – 2.0» = 1.0
[2 marks]
ionic bonding
OR
electrostatic forces between ions
«slight» movement brings ions of same charge adjacent to each other «causing the crystal to break»
OR
«slight» movement results in repulsion between layers «causing the crystal to break»
[2 marks]
Examiners report
The table summarizes some properties of graphite and graphene.

Graphene is two-dimensional, rather than three-dimensional, material.
Justify this by using the structure of graphene and information from the table.
Show that graphene is over 1600 times stronger than graphite.
Identify a value from the table which can be used to support the information about graphene given below.
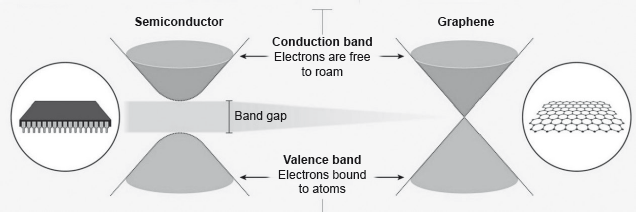
Electrons in a solid are restricted to certain ranges, or bands, of energy (vertical axis). In an insulator or semiconductor, an electron bound to an atom can break free only if it gets enough energy from heat or a passing photon to jump the “band gap”, but in graphene the gap is infinitely small.

Diamond, graphene, and graphite are all network solids.
Suggest, giving a reason, the electron mobility of diamond compared to graphene.
The melting point of diamond at 1 × 106 kPa is 4200 K (in the absence of oxygen).
Suggest, based on molecular structure, why graphene has a higher melting point under these conditions.
Markscheme
consists of single/one sheet/layer «of carbon atoms»
graphene has no density measurement
OR
graphene has no distance between layers data
OR
graphene has large specific surface area «compared to graphite»
Do not accept “sp2” alone without reference to single/one sheet/layer.
Accept “thickness of one atom” OR “consists of a plane” for M1.
[2 marks]
Any one of these alternatives:
ALTERNATIVE 1
«\(\frac{{1.3 \times {{10}^{11}}}}{{76 \times {{10}^6}}}\)»
1.7 × 103/1711
ALTERNATIVE 2
1600 × 76 × 106 = 1.2 × 1011 «is less than tensile strength of graphene»
ALTERNATIVE 3
\(\frac{{1.3 \times {{10}^{11}}}}{{1600}}\) = 8.1 × 107 «is greater than upper end of tensile strength for graphite»
Accept any value in the range 1700–27 083. Answer may be expressed in scientific notation or otherwise.
Accept any value calculated which is less than the graphene tensile strength based on a value chosen from within the 4.8–76 × 106 range.
[1 mark]
«graphene has a high electron mobility of» 15 000–200 000 «cm2 V–1 s–1»
A specific value or range of values must be given.
Accept any value in the 15 000–200 000 «cm2 V–1 s–1» range.
[1 mark]
smaller/zero
no delocalized electrons/electrons are bound/electrons not free to move/electrons not free to roam
OR
localized electrons «in sigma bonds»
OR
large band gap
Accept “diamond is a dielectric” OR “diamond does not conduct electricity” for M2.
Award [1 max] for just “immobile/less mobile”.
Award [2] for “electrons immobile «in diamond» due to the large band gap” OR "electrons «in diamond» immobile
since electrons are localized «in the sigma bonds»”.
[2 marks]
shorter bonds in graphene
OR
bonds in graphene intermediate between single and double
OR
bond order in graphene is 1.33
OR
delocalization creates stronger bonds
OR
shorter bonds are stronger
stronger/shorter bonds require higher temperature/faster thermal motion to be altered
OR
stronger/shorter bonds require greater energy to be broken
[2 marks]
Examiners report
Sunflower oil contains stearic, oleic and linoleic fatty acids. The structural formulas of these acids are given in section 34 of the data booklet.
Explain which one of these fatty acids has the highest boiling point.
10.0 g of sunflower oil reacts completely with 123 cm3 of 0.500 mol\(\,\)dm–3 iodine solution. Calculate the iodine number of sunflower oil to the nearest whole number.
Markscheme
stearic acid AND chain has no kinks/more regular structure
OR
stearic acid AND it has straight chain
OR
stearic acid AND no C=C/carbon to carbon double bonds
OR
stearic acid AND saturated
OR
stearic acid AND chains pack more closely together
stronger London/dispersion/instantaneous induced dipole-induced dipole forces «between molecules»
Accept “stearic acid AND greater surface area/electron density”.
M2 can only be scored if stearic acid is correctly identified.
Accept “stronger intermolecular/van der Waals’/vdW forces”.
[2 marks]
«n(I2) = 0.123 dm3 x 0.500 mol\(\,\)dm–3 =» 0.0615 «mol»
«m(I2) = 0.0615 mol x 253.8 g\(\,\)mol–1 =» 15.6 «g»
«iodine number \( = \frac{{15.6{\text{ g}} \times 100}}{{10.0{\text{ g}}}}\)» = 156
Award [3] for correct final answer.
Iodine number must be a whole number.
Award [2 max] for 78.
[3 marks]
Examiners report
Many lipids are found in the human body. One type of lipid is a triglyceride.
Steroids and phospholipids are both classes of lipid found in the body. Cholesterol is a steroid. A structure of lecithin, a phospholipid, is shown below.

The formulas of some fatty acids are shown in Table 22 of the Data Booklet. State the equation for the reaction between glycerol and stearic acid to form a triglyceride.
Compare the structures of the two fatty acids: linoleic and linolenic acids.
State why these two fatty acids are so important in the human diet.
Distinguish between HDL and LDL cholesterol.
Compare the composition of cholesterol with a phospholipid such as lecithin.
Determine whether cholesterol or lecithin is more soluble in water.
Markscheme

correct structure of glycerol and correct formula of stearic acid;
correct structure of triglyceride;
\({\text{3}}{{\text{H}}_{\text{2}}}{\text{O}}\) and coefficient of 3 on stearic acid;
Accept displayed or condensed formulas for molecules.
both have first double bond on C9 with carbon / linoleic has an \(\omega - 6{\text{ C=C}}\) double bond and linolenic acid has an \(\omega - 3{\text{ C=C}}\) double bond;
linoleic acid has 2 double carbon bonds and linolenic acid 3 double carbon bonds;
fatty acids are essential / body cannot synthesize them / OWTTE;
LDL is (a) larger (molecule) than HDL;
LDL transports cholesterol to arteries and HDL removes cholesterol from arteries;
LDL produced from saturated fats/trans fatty acids;
LDL increases the risk of heart disease/problems;
Accept converse statements for HDL.
Do not accept LDL is bad cholesterol and HDL is good cholesterol.
cholesterol is composed of C, H and O only and phospholipid contains C, H, O, P and N;
lecithin;
Examiners report
Candidates could not write an equation for the reaction between glycerol and stearic acid to form a triglyceride. Where candidates did write the correct equation they often did not balance the equation correctly.
In part (b) many candidates did not correctly recognize the difference in the number of carbon – carbon double bonds in the two fatty acids, nor the location of the double bonds and hence the significance of the omega-3 and omega-6 terminology.
Some candidates correctly identified that these fatty acids cannot be synthesised by the body and hence are essential.
In part (c) candidates could not distinguish between HDL and LDL, often referring simply and inadequately to ‘good’ and ‘bad’ cholesterol. Candidates had great difficulty comparing the composition of cholesterol with lecithin. An elemental comparison was required.
In part (c) candidates could not distinguish between HDL and LDL, often referring simply and inadequately to ‘good’ and ‘bad’ cholesterol. Candidates had great difficulty comparing the composition of cholesterol with lecithin. An elemental comparison was required.
In part (c) candidates could not distinguish between HDL and LDL, often referring simply and inadequately to ‘good’ and ‘bad’ cholesterol. Candidates had great difficulty comparing the composition of cholesterol with lecithin. An elemental comparison was required.
Aluminium and iron are both widely used in modern society.
Almost all iron is used in the form of an alloy. State the name of the most common type of iron alloy and the other element that is an essential component of these alloys.
Name:
Other element:
An early alloy of aluminium was Duralumin which contained small quantities of copper and magnesium. This is stronger and more rigid than pure aluminium. Explain on an atomic level why the addition of other elements has this effect.
Markscheme
Name: steel and other element: carbon;
atoms/ions of the alloying element are a different size/larger/smaller;
prevents the layers of atoms/ions sliding across each other;
Examiners report
This question was generally answered correctly.
Part (d) proved to be most challenging although it deals with the fundamental idea of the behaviour of alloys.
Linoleic acid is an essential fatty acid whose formula is given in Table 22 of the Data Booklet. Determine the mass of iodine, I2, which reacts with 100 g of linoleic acid.
Fats, such as butter, are solid triglycerides. Explain why fats have a higher energy value than carbohydrates.
The formula of stearic acid is also given in Table 22 of the Data Booklet. Explain why linoleic acid has a lower melting point compared to stearic acid.
Markscheme
2 mol of iodine reacts with 1 mol of linoleic acid;
\({M_{\text{r}}} = {\text{253.80 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) for iodine and \({M_{\text{r}}} = {\text{280.50 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) for linoleic acid;
\(\left( {\frac{{(507.60 \times 100.00)}}{{280.50}} = } \right){\text{ }}180.96{\text{ (g)}}/181{\text{ (g)}}\);
Award [3] for correct final answer.
Allow 254 g mol–1 for iodine and 281/280 g\(\,\)mol–1 for linoleic acid.
Award [2 max] for incorrect ratio calculation to give answers such as 90.4, 90.481, 90.7 (g) depending on Mr values used.
less oxidized (compared to carbohydrates) / fewer oxygen atoms (compared to carbohydrates);
C=C’s in linoleic acid cause the chain to be more uneven/kinked;
linoleic acid cannot pack as closely as stearic acid;
intermolecular/van der Waal’s/London/dispersion forces weaker in linoleic acid;
Accept converse argument for stearic acid.
Examiners report
In (a) the vast majority of candidates did not recognise that linoleic acid had two C=C double bonds and hence the ratio n(I2):n(acid) = 2:1. Candidates also made careless errors in calculating the molar mass of either/both iodine or/and linoleic acid.
Part (b) was answered well by about half of the candidates.
In Part(c) many candidates scored both marks, but there were cases which indicated poor preparation of candidates on this rather trivial question which appears so often in examinations.
Materials science involves understanding the properties of materials and applying those properties to desired structures.
Magnesium oxide, MgO, and silicon carbide, SiC, are examples of ceramic materials. State the name of the predominant type of bonding in each material.
Predict the predominant type of bonding for a binary compound AB in which the electronegativity of both atoms is low. Use section 29 of the data booklet.
Markscheme
MgO: ionic AND SiC: covalent
Accept “covalent network/network covalent” for “covalent” but not just “network”.
metallic «bonding»
Examiners report
The formula of linoleic acid is given in Table 22 of the Data Booklet.
Identify the structural formula of the triglyceride formed when three molecules of linoleic acid react with one molecule of glycerol (propane-1,2,3-triol), \({\text{C}}{{\text{H}}_{\text{2}}}{\text{OHCHOHC}}{{\text{H}}_{\text{2}}}{\text{OH}}\).
State the other product formed during this reaction.
Explain why the triglyceride formed from linoleic acid and glycerol is a liquid and not a solid at room temperature.
Describe how the triglyceride formed from linoleic acid and glycerol could be converted into a saturated fat and give any necessary conditions.
Other than the fact that it is a solid at room temperature, discuss two advantages and two disadvantages of a saturated fat compared to an unsaturated fat or oil.
Advantages:
Disadvantages:
Markscheme
 ;
;
Do not accept R for C17H31.
Penalize for incorrect bond connectivity.
water/\({{\text{H}}_{\text{2}}}{\text{O}}\);
double bonds cause a “kink” in the hydrocarbon chain / unsaturated hydrocarbon chains cannot pack so closely together (as saturated);
attractive forces/London/dispersion/van der Waals/vdW/LDF/ /instantaneous/temporary induced dipole-induced dipole forces between the molecules are weaker / less energy required to overcome the attraction between the molecules;
(d) addition of hydrogen/\({{\text{H}}_{\text{2}}}\) / hydrogenation;
heat and catalyst/Zn/Cu/Ni/Pd/Pt;
Accept any temperature in range 140–225 °C.
Advantages:
decreases rate of oxidation / makes it more stable / slows rancidification / has longer shelf life;
greater energy released per gram / OWTTE;
controls hardness/plasticity/stiffness;
Disadvantages:
increase risk of heart disease / increase low-density/LDL cholesterol;
does not contain essential/omega-3/omega-6 fatty acids;
hydrogenated fats might contain trans-fatty acids;
Examiners report
Generally candidates struggled with the details of the triglyceride structure; very few were able to gain the mark for part (a). Almost all the candidates were able to state water as the other product and gain the mark in part (b). Candidates often missed the specific details for the answer to part (c); they were able to identify the presence of double bonds or unsaturation but had difficulty expanding and linking the idea to how this changed packing and intermolecular forces. For part (d) many candidates stated hydrogenation as the answer and scored one point but many were not able to state the two reaction conditions correctly. Most responses on advantages and disadvantages of saturated fats, part (e), were too general for example, affects your health. A common statement was ‘better storage/ transport’ as an advantage for saturated fats. Most candidates only scored one or two points for this question.
Generally candidates struggled with the details of the triglyceride structure; very few were able to gain the mark for part (a). Almost all the candidates were able to state water as the other product and gain the mark in part (b). Candidates often missed the specific details for the answer to part (c); they were able to identify the presence of double bonds or unsaturation but had difficulty expanding and linking the idea to how this changed packing and intermolecular forces. For part (d) many candidates stated hydrogenation as the answer and scored one point but many were not able to state the two reaction conditions correctly. Most responses on advantages and disadvantages of saturated fats, part (e), were too general for example, affects your health. A common statement was ‘better storage/ transport’ as an advantage for saturated fats. Most candidates only scored one or two points for this question.
Generally candidates struggled with the details of the triglyceride structure; very few were able to gain the mark for part (a). Almost all the candidates were able to state water as the other product and gain the mark in part (b). Candidates often missed the specific details for the answer to part (c); they were able to identify the presence of double bonds or unsaturation but had difficulty expanding and linking the idea to how this changed packing and intermolecular forces. For part (d) many candidates stated hydrogenation as the answer and scored one point but many were not able to state the two reaction conditions correctly. Most responses on advantages and disadvantages of saturated fats, part (e), were too general for example, affects your health. A common statement was ‘better storage/ transport’ as an advantage for saturated fats. Most candidates only scored one or two points for this question.
Generally candidates struggled with the details of the triglyceride structure; very few were able to gain the mark for part (a). Almost all the candidates were able to state water as the other product and gain the mark in part (b). Candidates often missed the specific details for the answer to part (c); they were able to identify the presence of double bonds or unsaturation but had difficulty expanding and linking the idea to how this changed packing and intermolecular forces. For part (d) many candidates stated hydrogenation as the answer and scored one point but many were not able to state the two reaction conditions correctly. Most responses on advantages and disadvantages of saturated fats, part (e), were too general for example, affects your health. A common statement was ‘better storage/ transport’ as an advantage for saturated fats. Most candidates only scored one or two points for this question.
Generally candidates struggled with the details of the triglyceride structure; very few were able to gain the mark for part (a). Almost all the candidates were able to state water as the other product and gain the mark in part (b). Candidates often missed the specific details for the answer to part (c); they were able to identify the presence of double bonds or unsaturation but had difficulty expanding and linking the idea to how this changed packing and intermolecular forces. For part (d) many candidates stated hydrogenation as the answer and scored one point but many were not able to state the two reaction conditions correctly. Most responses on advantages and disadvantages of saturated fats, part (e), were too general for example, affects your health. A common statement was ‘better storage/ transport’ as an advantage for saturated fats. Most candidates only scored one or two points for this question.
Suggest, in terms of its structure, why vitamin D is fat-soluble using section 35 of the data booklet.
Markscheme
«mostly» non-polar
OR
hydrocarbon backbone
OR
only 1 hydroxyl «group so mostly non-polar»
Accept “alcohol/hydroxy” for “hydroxyl” but not “hydroxide”.
[1 mark]
Examiners report
The structures of morphine, diamorphine and codeine are given in section 37 of the data booklet.
Explain why diamorphine passes more readily than morphine through the blood-brain barrier.
Suggest a reagent used to prepare diamorphine from morphine.
Suggest one reason why codeine is available without prescription in some countries whilst morphine is administered under strict medical supervision.
Markscheme
Any two of:
diamorphine has ester/ethanoate/acetate «groups» AND morphine has hydroxyl «groups»
diamorphine/ester/ethanoate/acetate groups less polar
diamorphine more soluble in lipids
Accept “alcohol/hydroxy” for “hydroxyl” but not “hydroxide”.
Accept “diamorphine non-polar”.
Accept converse statements.
[2 marks]
ethanoic/acetic anhydride
OR
ethanoyl/acetyl chloride
Accept other possible reagents, such as ethanoic/acetic acid or acetyl bromide.
Accept chemical formulas.
[1 mark]
morphine has a smaller therapeutic window
Accept converse statements.
Accept “codeine has lower activity” OR “codeine has lower risk of overdose” OR “codeine is less potent”.
Do not accept “lower abuse potential for codeine” OR “codeine less addictive” OR “codeine has a lower bioavailability”.
[1 mark]
Examiners report
Vitamins can be water-soluble or fat-soluble.
Explain, at the molecular level, why vitamin D is soluble in fats. Use section 35 of the data booklet.
State one function of vitamin D in the body.
Markscheme
«mainly» hydrocarbon/non-polar «structure»
forms London/dispersion/instantaneous induced dipole-induced dipole forces «with fats»
Accept “forms van der Waals’/vdW forces”.
Award [1 max] for “contains only one OH/hydroxyl AND cannot form «enough» H-bonds”.
helps absorb calcium
OR
helps build bones
OR
helps keep bones healthy
OR
helps block the release of parathyroid hormone
OR
helps in muscle function
OR
helps immune system function
OR
cell growth
OR
reduction of inflammation
OR
protection from osteoporosis
OR
prevents rickets
Accept helps prevent colon/breast/prostate cancer.
Accept treat/prevent diabetes/heart disease/high blood pressure/multiple sclerosis.
Accept other correct answers.
Examiners report
The mild analgesic aspirin can be prepared in the laboratory from salicylic acid.
(CH3CO)2O + HOC6H4COOH → CH3CO2C6H4COOH + CH3COOH
Salicylic acid Aspirin
After the reaction is complete, the product is isolated, recrystallized, tested for purity and the experimental yield is measured. A student’s results in a single trial are as follows.
Literature melting point data: aspirin = 138–140 °C
Determine the percentage experimental yield of the product after recrystallization. The molar masses are as follows: M(salicylic acid) = 138.13 g mol−1, M(aspirin) = 180.17 g mol−1. (You do not need to process the uncertainties in the calculation.)
Suggest why isolation of the crude product involved the addition of ice-cold water.
Justify the conclusion that recrystallization increased the purity of the product, by reference to two differences between the melting point data of the crude and recrystallized products.
State why aspirin is described as a mild analgesic with reference to its site of action.
Markscheme
ALTERNATIVE 1:
«theoretical yield = \(\frac{{1.552\,{\text{g}}}}{{138.13\,{\text{g}}\,{\text{mo}}{{\text{l}}^{ - 1}}}}\) × 180.17 g mol−1 =» 2.024 «g»
«experimental yield = \(\frac{{1.124{\rm{g}}}}{{2.024{\rm{g}}}}\) × 100 =» 55.53 «%»
ALTERNATIVE 2:
«\(\frac{{1.552\,{\text{g}}}}{{138.13\,{\text{g}}\,{\text{mo}}{{\text{l}}^{ - 1}}}}\)»= 0.01124 «mol salicylic acid/aspirin theoretical» AND
«\(\frac{{1.124\,{\text{g}}}}{{180.17\,{\text{g}}\,{\text{mo}}{{\text{l}}^{ - 1}}}}\)»= 0.006239 «mol aspirin experimental»
«experimental yield = \(\frac{{0.006239{\rm{mol}}}}{{0.01124{\rm{mol}}}}\) x 100 =» 55.51 «%»
Accept answers in the range 55.4 % to 55.7 %.
Award [2] for correct final answer.
low temperature gives greater difference between solubility of aspirin and impurities
OR
«product» crystallizes out from cold solution/«ice-cold water/lower temperature» speeds up crystallization process
OR
aspirin/product has low solubility «in water» at low temperatures
intercepts pain stimulus at source/acts at site of pain
OR
interferes with production of pain sensitizing substances/prostaglandins «at site of pain»
Examiners report
recrystallized melting point is higher
OR
recrystallized melting point is closer to pure substance/literature value
smaller range of values
Polymers are made up of repeating monomer units which can be manipulated in various ways to give structures with desired properties.
(i) Draw the structure of 2-methylpropene.
(ii) Deduce the repeating unit of poly(2-methylpropene).
Deduce the percentage atom economy for polymerization of 2-methylpropene.
(i) Suggest why incomplete combustion of plastic, such as polyvinyl chloride, is common in industrial and house fires.
(ii) Phthalate plasticizers such as DEHP, shown below, are frequently used in polyvinyl chloride.
With reference to bonding, suggest a reason why many adults have measurable levels of phthalates in their bodies.
Markscheme
i
OR
H2C=C(CH3)2
ii
OR
−CH2C(CH3)2−
Continuation bonds needed for mark.
No penalty if square brackets present or “n” appears after the bracket/formula.
«same mass of product as reactant, thus» 100«%»
Accept “less than 100%” only if a reason is given (eg, the catalyst is not converted into the product, or other reasonable answer).
i
due to stability of plastics/strong covalent bonds
OR
low volatility preventing good mixing with oxygen «gas»
OR
lack of/insufficient oxygen
OR
plastics are often parts of devices with non-combustible components «which mechanically prevent the combustion of plastic components»
OR
PVC already partly oxidised «because some C–H bonds are replaced with C–Cl bonds», so it cannot produce enough heat for complete combustion
OR
many industrial/household materials contain additives that reduce their flammability/act as flame retardants
ii
weakly bound to the PVC/no covalent bonds to PVC/only London/dispersion/instantaneous induced dipole-induced dipole forces between DEHP and PVC AND leach/evaporate «from PVC» to atmosphere/food chain
OR
has low polarity/contains non-polar hydrocarbon chains AND fat-soluble/deposits in the fatty tissues
OR
has unusual structural fragments/is a xenobiotic/difficult to metabolise AND stays in the body for a long time
Examiners report
Peptidase enzyme in the digestive system hydrolyses peptide bonds.
A tripeptide Ala-Asp-Lys was hydrolysed and electrophoresis of the mixture of the amino acids was carried out at a pH of 6.0. Refer to section 33 of the data booklet.
Identify the type of metabolic process that occurs in the hydrolysis of the peptide during digestion.
Identify the name of the amino acid that does not move under the influence of the applied voltage.
Deduce, giving a reason, which amino acid will develop closest to the negative electrode.
The breakdown of a dipeptide in the presence of peptidase was investigated between 18 °C and 43 °C. The results are shown below.
Comment on the rate of reaction at temperature X in terms of the enzyme’s active site.
The solubility of a vitamin depends on its structure.
Identify the vitamin given in section 35 of the data booklet that is the most soluble in water.
Pollution from heavy metal ions has become a health concern.
Outline how the presence of heavy metal ions decreases the action of enzymes.
Outline how lead ions could be removed from an individual suffering from lead poisoning.
Markscheme
catabolism/catabolic
[1 mark]
alanine
Do not accept ala.
[1 mark]
Lys/lysine
pH «buffer» < pI «Lys»
OR
buffer more acidic than Lys «at isoelectric point»
OR
«Lys» exists as
OR
«Lys» charged positively/has +1/1+ «overall» charge «and moves to negative electrode»
Do not apply ECF from M1.
Accept converse argument.
Do not accept just “has H3N+ group” for M2 (as H3N+ is also present in zwitterion).
Do not penalize if COOH is given in the structure of lysine at pH 6 instead of COO–.
[2 marks]
highest frequency of successful collisions between active site and substrate
OR
highest frequency of collisions between active site and substrate with sufficient energy/\(E \geqslant {E_{\text{a}}}\) AND correct orientation/conformation
OR
optimal shape/conformation of the active site «that matches the substrate»
OR
best ability of the active site to bind «to the substrate»
Accept “number of collisions per unit time” for “frequency”.
Do not accept “all active sites are occupied”.
[1 mark]
ascorbic acid/vitamin C
[1 mark]
react/bind/chelate with enzyme
OR
disrupt ionic salt bridges
OR
affect shape of tertiary/quaternary structures
OR
precipitate enzymes
OR
break/disrupt disulfide bridges/bonds
Do not accept “changes shape of active site” by itself.
[1 mark]
«use of» host-guest chemistry
OR
chelation «therapy»
Accept specific medication/chelating agent such as EDTA, CaNa2 EDTA, succimer, D-penicillamine, dimercaprol.
[1 mark]
Examiners report
Lipids and carbohydrates contain the same elements but have different properties.
List the building blocks of triglycerides and carbohydrates.
The drain pipe of a kitchen sink can become clogged by fatty acids, such as linoleic acid, C18H32O2, but not by the trisaccharide, raffinose, C18H32O16, containing the same number of carbon atoms.
Explain why raffinose is far more water soluble than linoleic acid.
Solid fat triglycerides can also clog kitchen sink drains.
Explain how sodium hydroxide unblocks the drain.
The amount of proteins, fats and carbohydrates determine the energy content of foods.
Explain why linoleic acid, C18H32O2, is a more efficient energy storage molecule than raffinose, C18H32O16.
Markscheme
Triglycerides:
organic acid/fatty acid and glycerol/propane-1,2,3-triol
AND
Carbohydrates:
monosaccharides
Accept simple sugars.
[1 mark]
«water/aqueous solubility depends on forming many» H-bonds with water
raffinose has many hydroxyl/O–H/oxygen atoms/O «and forms many H-bonds» AND linoleic acid has few/one hydroxyl/O–H/oxygen atom/O/carboxyl group/ COOH/is largely non-polar «and cannot form many H-bonds»
Accept statement which implies comparison.
[2 marks]
«base» hydrolysis/saponification
OR
«produces glycerol and» soap/salt of the «fatty» acid
«products are» water soluble «and drain away»
Accept condensed formulas.
Accept non-balanced equation.
Accept “RCOONa”.
[2 marks]
linoleic acid/C18H32O2 combustion/oxidation more exothermic «per mol»
linoleic acid/C18H32O2 has lower proportion/number of O atoms
OR
linoleic acid/C18H32O2 is less oxidized
Accept converse arguments.
[2 marks]
Examiners report
Lactose is a disaccharide formed by the condensation reaction of the monosaccharides galactose and glucose.
Describe what is meant by a condensation reaction.
Draw the structure of galactose on the skeleton provided.
Explain how the inclusion of carbohydrates in plastics makes them biodegradable.
Markscheme
«reaction in which» two reactants/molecules/functional groups bond/react «to form a larger molecule/single main product»
small/tiny molecule
OR
H2O formed
Accept formula or name of a specified small molecule other than water such as ammonia, ethanoic/acetic acid,
ethanol, hydrogen sulfide etc. for M2.
Do not accept just “molecule formed”.
Award [1 max] for an example giving an equation of a condensation reaction such as the formation of a disaccharide.
Accept “alpha” or “beta” form of galactose.
Any two of:
makes the plastic more hydrophilic/water soluble
carbohydrates are broken down/hydrolysed by bacteria/microorganisms
makes plastic more accessible to bacteria as holes/channels are created
OR
plastic of lower density is more permeable/susceptible to water/oxygen/heat/pressure
weakens intermolecular/London/dispersion/instantaneous induced dipole-induced dipole forces «between polymer chains in the plastic»
Accept “van der Waals/vdW” for “London” forces.
[Max 2 Marks]
Examiners report
Solubility plays an important role in the bioavailability of drugs in the body.
Suggest why aspirin is slightly soluble in water. Refer to section 37 of the data booklet.
Formulate an equation for the conversion of aspirin to a more water soluble derivative.
A student prepares aspirin from salicylic acid in the laboratory, extracts it from the reaction mixture, ensures the sample is dry and determines its melting point.
Suggest why the melting point of the student’s sample is lower and not sharp compared to that of pure aspirin.
Organic molecules can be characterized using infrared (IR) spectroscopy.
Compare and contrast the infrared peaks above 1500 cm−1 in pure samples of aspirin and salicylic acid using section 26 of the data booklet.
The pharmaceutical industry is one of the largest producers of waste solvents.
State a green solution to the problem of organic solvent waste.
Markscheme
presence of «large» benzene/arene ring AND non-polar/hydrophobic
OR
presence of «large» benzene/arene ring AND cannot form H-bond with water
contain COOH/carboxyl/–OH/hydroxyl «and ester group» AND polar/hydrophilic
OR
contain COOH/carboxyl/–OH/hydroxyl «and ester group» AND can form H-bonds with water
Accept “phenyl” for “benzene ring”.
Accept "carboxylic acid" for "carboxyl".
Do not accept "alcohol" for "hydroxyl".
[2 marks]
OR
C6H4(OCOCH3)COOH + NaOH → C6H4(OCOCH3)COONa + H2O
Charges (O– and Na+) not necessary to score the mark.
Accept net ionic equation.
Accept any strong base in place of NaOH.
[1 mark]
«student’s» sample impure
lattice disrupted/not uniform «due to presence of impurities»
OR
fewer interparticle/intermolecular forces «due to presence of impurities»
Accept converse arguments.
[2 marks]
One similarity:
peak at 2500–3000 «cm–1»/peak due to O–H/hydroxyl in carboxylic acids
OR
peak at 1700–1750 «cm–1»/peak due to C=O/carbonyl
OR
peak at 2850–3090 «cm–1»/peak due to C–H of arene
One difference:
peak at 3200–3600 «cm–1» in salicylic acid/ peak due to O–H in phenol in salicylic acid
OR
«two» peaks at 1700–1750 «cm–1» in aspirin AND one peak «in the same area» in salicylic acid
Accept “peak at 1600 cm–1 for arene/benzene ring” – not in the data booklet.
Accept “2500–3600 cm–1 «overlapping absorptions of two O–H» in salicylic acid”.
Accept “stronger/broader/split peak at 1700–1750 cm–1 in aspirin”.
[2 marks]
«use of» alternative solvents such as supercritical/liquid CO2
OR
use of water «as solvent»
OR
solvent-free reactions «for example, polymerization of propene»
OR
solid-state chemistry
OR
recycle «waste» solvents
OR
catalysis that leads to better/higher yield
OR
reducing number of steps
Do not accept political/regulatory solutions.
“catalysis” not sufficient for mark.
[1 mark]
Examiners report
Lipids are an important part of the human diet.
Fatty acids react with glycerol to form fats and oils. State the name of the chemical link formed in this reaction and the name of the other product.
The table below shows average figures for the percentage fatty acid composition of some common fats and oils.
(i) Deduce, with a reason, which fat or oil from the table above has the lowest iodine number.
(ii) Deduce, with a reason, which fat or oil from the table above is most likely to become rancid when exposed to the air.
(iii) The P/S index of a fat or oil is the ratio of polyunsaturated fat to saturated fat present. It is sometimes used to compare the relative health benefits of different lipids in the diet. Calculate the P/S index of beef fat and soybean oil.
(iv) Suggest why a P/S index of greater than 1 is considered beneficial to health.
(v) Cotton seed oil and corn oil have similar iodine numbers but the melting point of cotton seed oil is higher than that of corn oil. Suggest an explanation in terms of the structure and bonding in these two oils.
Markscheme
Name of the chemical link: ester/ethoxycarbonyl
AND
Name of the other product: water
Do not accept formulas.
Do not accept “esterification”
i
coconut oil AND lowest «percentage of» unsaturated fatty acids
OR
coconut oil AND smallest number of C=C bonds
OR
coconut oil AND highest «percentage of» saturated fatty acids
Accept “fats” for “fatty acids”.
ii
soybean oil AND highest «percentage of» polyunsaturated fatty acids
OR
soybean oil AND greatest number of C=C bonds
OR
soybean oil AND lowest «percentage of» saturated fatty acids
Accept “fats” for “fatty acids”.
iii
Beef fat: «P/S = \(\frac{3}{{59}}\) = » 0.05
AND
Soybean oil: «P/S = \(\frac{{50 + 8}}{{14}}\) =» 4.1
iv
«higher proportion of» polyunsaturated fatty acids decrease risk of atherosclerosis/heart disease/cardiovascular disease/CVD
OR
«higher proportion of» polyunsaturated fatty acids which are less likely to be deposited on the walls of arteries «than saturated fatty acids»
Accept converse arguments.
Accept correct arguments in terms of HDL and LDL but not in terms of “good” and “bad” cholesterol.
Accept “fats” for “fatty acids”.
v
Any two of:
cotton seed oil has «a higher proportion of» longer chain/greater molar mass fatty acids
molecules of cotton seed oil have greater surface area/have higher electron density
Accept “molecules of cotton seed oil are packed more closely/have more regular structure” for M2.
stronger London/dispersion/instantaneous induced dipole-induced dipole forces between chains in cotton seed oil
Accept converse arguments.
Accept “fats” for “fatty acids”.
Examiners report
The development of materials with unique properties is critical to advances in industry.
Low density polyethene (LDPE) and high density polyethene (HDPE) are both addition polymers.
Outline two properties a substance should have to be used as liquid-crystal in a liquid-crystal display.
Describe how the structures of LDPE and HDPE affect one mechanical property of the plastics.
One of the two infrared (IR) spectra is that of polyethene and the other of polytetrafluoroethene (PTFE).
Deduce, with a reason, which spectrum is that of PTFE. Infrared data is given in section 26 of the data booklet.
Many plastics used to be incinerated. Deduce an equation for the complete combustion of two repeating units of PVC, (–C2H3Cl–)2.
Markscheme
Any two of:
ability to form a LC phase
chemically stable
«LC phase that is» stable over suitable temperature range
polar
OR
being able to change orientation with applied electric field
rapid switching speed «responds to changes of voltage quickly»
Accept “ability of molecules to transmit light under certain conditions” OR “rodshaped molecules” OR “stable to light/not light sensitive”.
[Max 2 Marks]
branching in LDPE prevents close packing «of chains»
LDPE is more flexible/less rigid
OR
LDPE has lower «tensile» strength
Do not accept “difference in density”.
Award [1 max] for stating “branching in LDPE AND little/no branching in HDPE”.
B AND absence «of absorption of» C–H at 2850–3090 «cm–1»
OR
B AND presence of «absorption of» C–F at 1000–1400 «cm–1»
(–C2H3Cl–)2 (s) + 5O2 (g) → 4CO2 (g) + 2H2O (l) + 2HCl (g)
correct species in reactants and products
balanced
Accept “(–C2H3Cl–)2 (s) + 5.5O2 (g) → 4CO2 (g) + 3H2O (l) + Cl2 (g)”.
Award M2 only if M1 correct.
Examiners report
Infrared (IR) spectroscopy is often used for the identification of polymers, such as PETE, for recycling.
LDPE and high density polyethene (HDPE) have very similar IR spectra even though they have rather different structures and physical properties.
Below are the IR spectra of two plastics (A and B); one is PETE, the other is low density polyethene (LDPE).
Deduce, giving your reasons, the identity and resin identification code (RIC) of A and B using sections 26 and 30 of the data booklet.
Describe the difference in their structures.
Explain why the difference in their structures affects their melting points.
Markscheme
A RIC: 1 AND B RIC: 4
ALTERNATIVE 1:
«only» PETE contains carbonyl/C=O/ester/COO groups
carbonyl groups absorb at 1700–1750 «cm–1»
ALTERNATIVE 2:
LDPE contains more C–H bonds «than PETE»
C–H bonds absorb at 2850–3090 «cm–1»
For either, accept specific frequencies in these ranges (eg 1735 «cm–1» or 2900 «cm–1»).
[3 marks]
HDPE less branched
OR
LDPE more branched
Accept “no branching in HDPE AND branching in LDPE”.
[1 mark]
HDPE «polymer» chains/molecules can pack together more closely «than LDPE chains»
OR
HDPE «polymer» chains/molecules have a higher contact surface area «than LDPE chains»
stronger intermolecular/dispersion/London/van der Waals’ forces in HDPE AND higher melting point
Accept converse arguments.
[2 marks]
Examiners report
Palmitic acid has a molar mass of 256.5 g mol−1.
The apparatus in the diagram measures the surface pressure created by palmitic acid molecules on the surface of water. This pressure is caused by palmitic acid molecules colliding with the fixed barrier. The pressure increases as the area, A, available to the palmitic acid is reduced by the movable barrier.

When a drop of a solution of palmitic acid in a volatile solvent is placed between the barriers, the solvent evaporates leaving a surface layer. The graph of pressure against area was obtained as the area A was reduced.

Part of this molecule is hydrophilic (bonds readily to water) and part hydrophobic (does not bond readily to water). Draw a circle around all of the hydrophilic part of the molecule.
When a small amount of palmitic acid is placed in water it disperses to form a layer on the surface that is only one molecule thick. Explain, in terms of intermolecular forces, why this occurs.
Suggest why there is a small increase in the surface pressure as the area is reduced to about 240 cm2, but a much faster increase when it is further reduced.
The solution of palmitic acid had a concentration of 0.0034 mol dm−3. Calculate the number of molecules of palmitic acid present in the 0.050 cm3 drop, using section 2 of the data booklet.
Assuming the sudden change in gradient occurs at 240 cm2, calculate the area, in cm2, that a single molecule of palmitic acid occupies on surface of the water.
If you did not obtain an answer for (b)(ii) use a value of 8.2 × 1016, but this is not the correct answer.
Markscheme

Must cut CH2–CO bond AND enclose all of the –COOH group.
[1 mark]
Any two of:
–COOH/CO/OH/carboxylate/carboxyl/hydroxyl/hydroxy group forms hydrogen bonds/H-bonds to water
London/dispersion/instantaneous induced dipole-induced dipole forces occur between hydrocarbon chains
hydrocarbon chain cannot form hydrogen bonds/H-bonds to water
strong hydrogen bonds/H-bonds between water molecules exclude hydrocarbon chains «from the body of the water»
Accept “hydrophilic part/group forms hydrogen bonds/H-bonds to water”.
Accept “hydrophobic section” instead of “hydrocarbon chain”.
Award [1 max] for answers based on “the –COOH group being polar AND the hydrocarbon chain being non-polar”.
[2 marks]
Above about 240 cm2:
greater collision frequency/collisions per second between «palmitic acid» molecules and the barrier «as area reduced»
At less than about 240 cm2:
molecules completely cover the surface
OR
there is no space between molecules
OR
force from movable barrier transmitted directly through the molecules to the fixed barrier
OR
«palmitic acid» molecules are pushed up/down/out of layer
For both M1 and M2 accept “particles” for “molecules”.
For M1 accept “space/area between molecules reduced” OR “molecules moving closer together”.
[2 marks]
amount of acid = «5.0 × 10–5 dm3 × 0.0034 mol dm–3» = 1.7 × 10–7 «mol»
number of molecules = «1.7 × 10–7 mol × 6.02 × 1023 mol–1 =» 1.0 × 1017
Award [2] for correct final answer.
Award [1] for “1.0 × 1020”.
[2 marks]
«area = \(\frac{{240{\text{ c}}{{\text{m}}^2}}}{{1.0 \times {{10}^{17}}}}\) » 2.4 × 10–15 «cm2»
[1 mark]